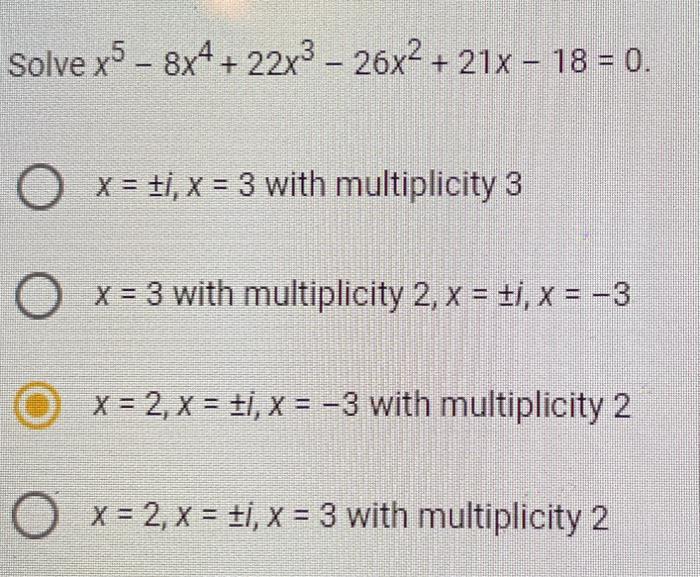- Accueil
- 21x 22x
- c) 20,000 200000 13. An element, X, have three isotopes 22X. The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be ((a) 9% (b) 8% (
c) 20,000 200000 13. An element, X, have three isotopes 22X. The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be ((a) 9% (b) 8% (
4.7 (400) · € 29.99 · En Stock
Click here:point_up_2:to get an answer to your question :writing_hand:c 20000let 20000013 an element x have three isotopes22x the percentage abundance ofits average atomic
Click here👆to get an answer to your question ✍️ -c- 20-000 200000 13- An element- X- have three isotopes 22X- The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be -a- 9- -b- 8- -c- 10- -d- 0- have three isotopes 20X- 21X and age abundance of 20X is 90- and c mass of the element is 20-11-The

1.What information would you need to calculate the average atomic

The relative abundance of two isotopes of atomic masses 85 and 87

Finding Percent Abundance (3 Isotopes)

PDF) Precalculus Seventh Edition With the assistance of David C

The three stable isotopes of neon `Ne^20, Ne^21 ` and `Ne^22` have

An element X exist in three isotopic forms. If percentage

An element, X, have three isotopes X X and X”. The percentage
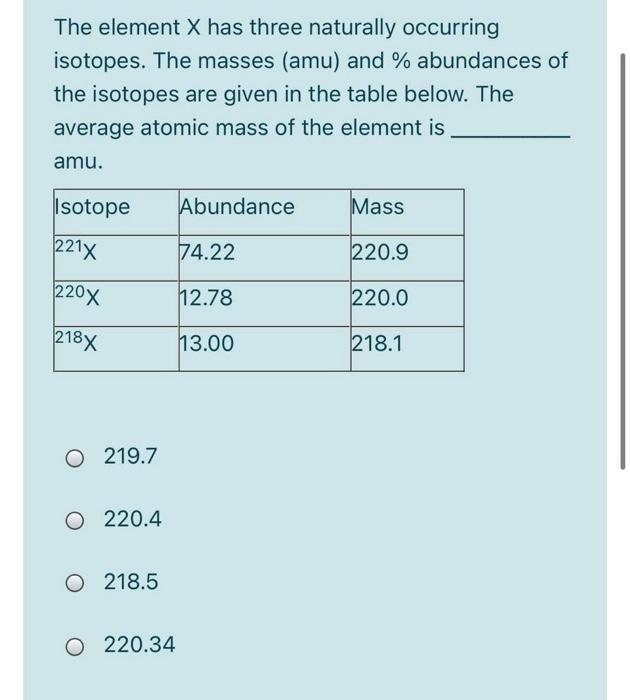
Solved The element X has three naturally occurring isotopes

GRB Physical Chemistry IIT JEE 3, PDF

Q An element x has three isotopes x20,x21,x22. The percentage

Finding Percent Abundance (3 Isotopes)